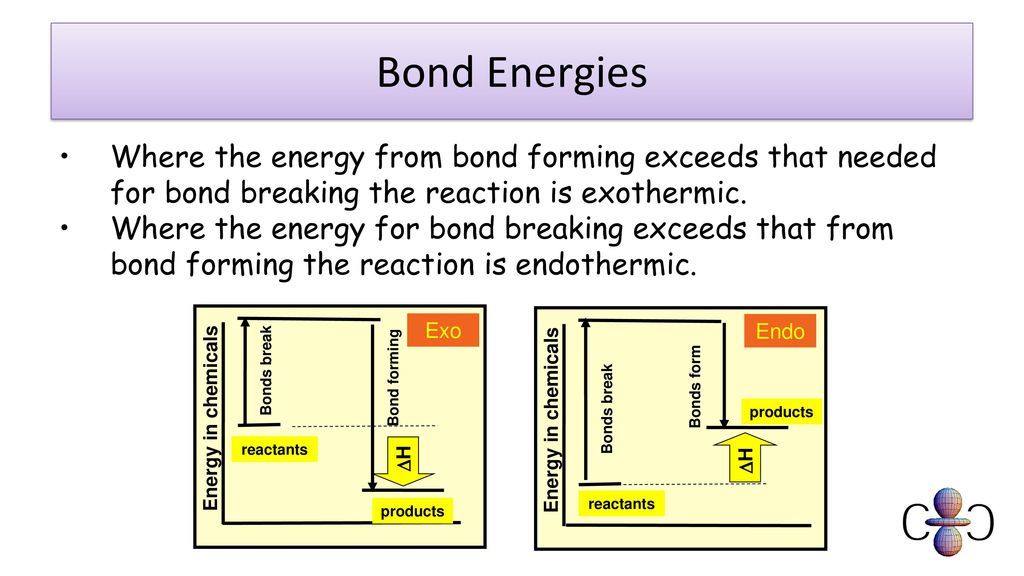
Is Forming Bonds Exothermic - The iupac definition of exothermic doesn't make any reference to bond formation. 10 is bond formation strictly exothermic? Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies. If so, why don't helium form bonds with neon? The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and.
Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies. The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and. If so, why don't helium form bonds with neon? 10 is bond formation strictly exothermic? The iupac definition of exothermic doesn't make any reference to bond formation.
Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies. The iupac definition of exothermic doesn't make any reference to bond formation. The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and. 10 is bond formation strictly exothermic? If so, why don't helium form bonds with neon?
Energetics. ppt download
If so, why don't helium form bonds with neon? The iupac definition of exothermic doesn't make any reference to bond formation. The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and. Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum.
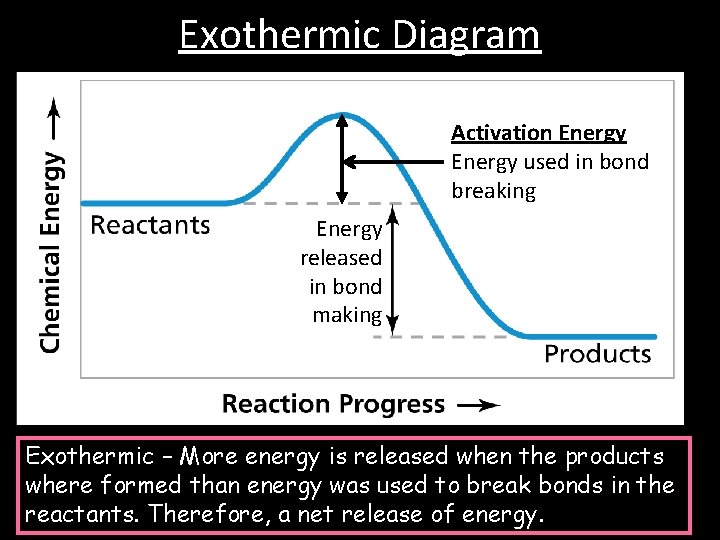
Exothermic and Endothermic Reactions Energy and Chemical Reactions
Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies. The iupac definition of exothermic doesn't make any reference to bond formation. 10 is bond formation strictly exothermic? The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and..
Bonding Between Atoms Why Do Atoms Form Bonds
If so, why don't helium form bonds with neon? 10 is bond formation strictly exothermic? Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies. The iupac definition of exothermic doesn't make any reference to bond formation. The reaction is endothermic or exothermic depending on the difference between the.
5.1 Exothermic and endothermic reactions IGCSE and A Level Chemistry
The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and. The iupac definition of exothermic doesn't make any reference to bond formation. 10 is bond formation strictly exothermic? If so, why don't helium form bonds with neon? Whether energy is released or taken up (whether the reaction is exothermic.
PPT Energy and Chemical Reactions Energetics PowerPoint Presentation
The iupac definition of exothermic doesn't make any reference to bond formation. If so, why don't helium form bonds with neon? The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and. Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum.
THERMOCHEMISTRY Courtesy of ppt download
Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies. The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and. The iupac definition of exothermic doesn't make any reference to bond formation. If so, why don't helium form.
Cover Sheet Vocab Sheet ppt download
Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies. The iupac definition of exothermic doesn't make any reference to bond formation. The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and. 10 is bond formation strictly exothermic?.
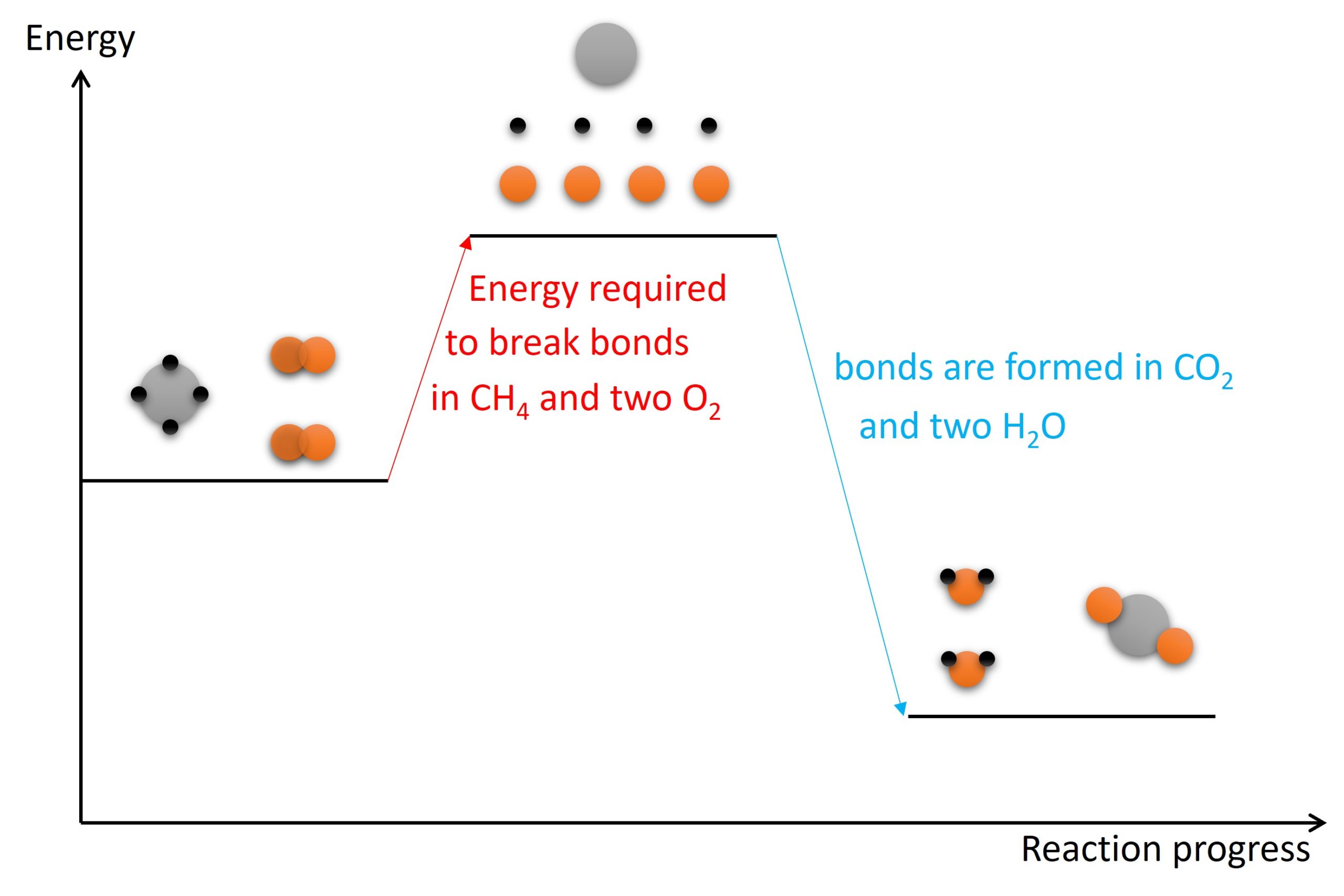
Energy changes in chemistry bond enthalpies ppt download
The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and. 10 is bond formation strictly exothermic? Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies. The iupac definition of exothermic doesn't make any reference to bond formation..
Bonding Between Atoms Why Do Atoms Form Bonds
Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies. The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and. If so, why don't helium form bonds with neon? 10 is bond formation strictly exothermic? The iupac definition.
Ch. 7 Notes Day 4 4/25/ ppt download
If so, why don't helium form bonds with neon? Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies. 10 is bond formation strictly exothermic? The iupac definition of exothermic doesn't make any reference to bond formation. The reaction is endothermic or exothermic depending on the difference between the.
The Iupac Definition Of Exothermic Doesn't Make Any Reference To Bond Formation.
10 is bond formation strictly exothermic? If so, why don't helium form bonds with neon? The reaction is endothermic or exothermic depending on the difference between the total energy released by the forming of bonds and. Whether energy is released or taken up (whether the reaction is exothermic or endothermic) depends on the sum of the energies.


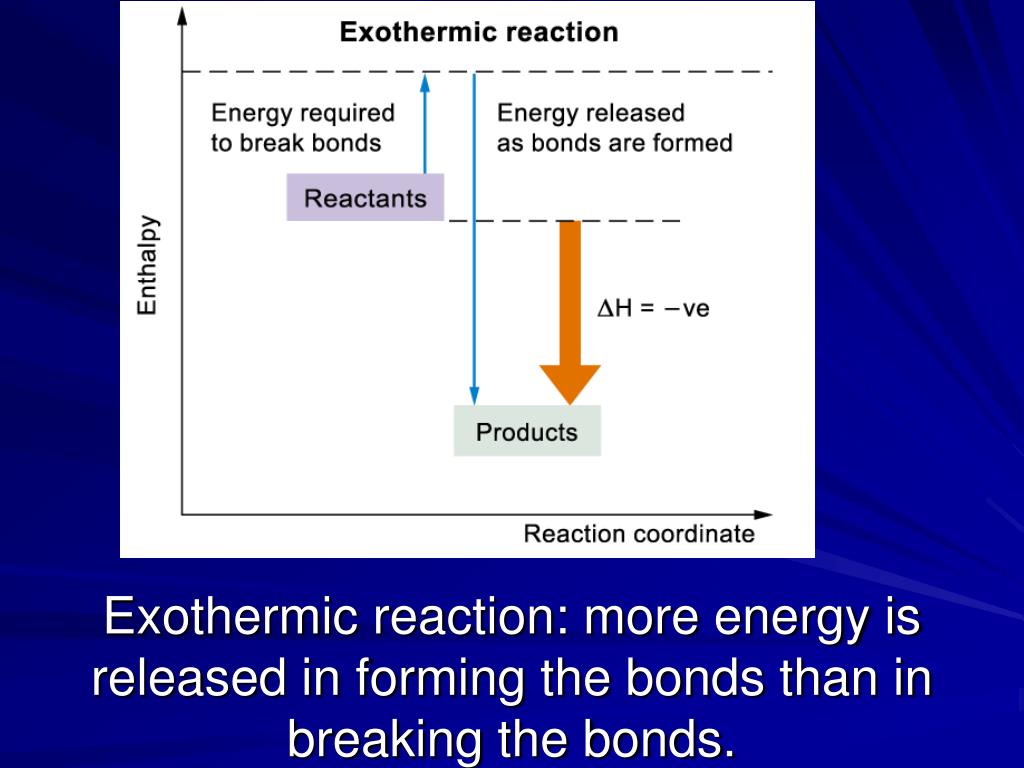
+than+those+of+the+reactants..jpg)