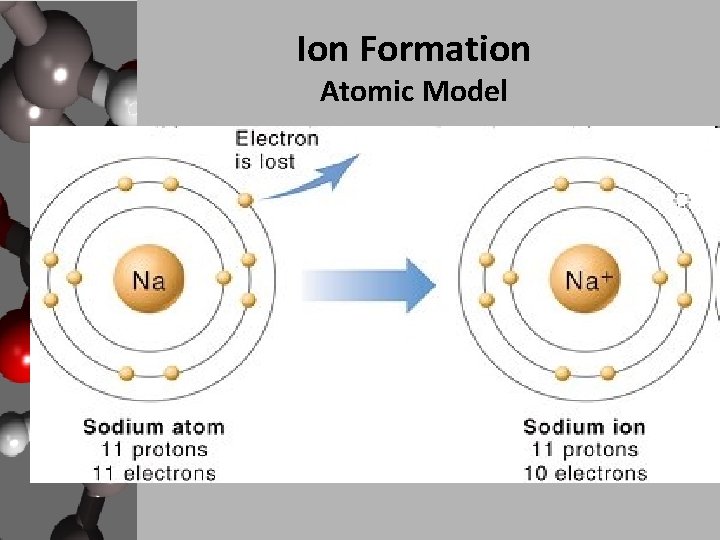
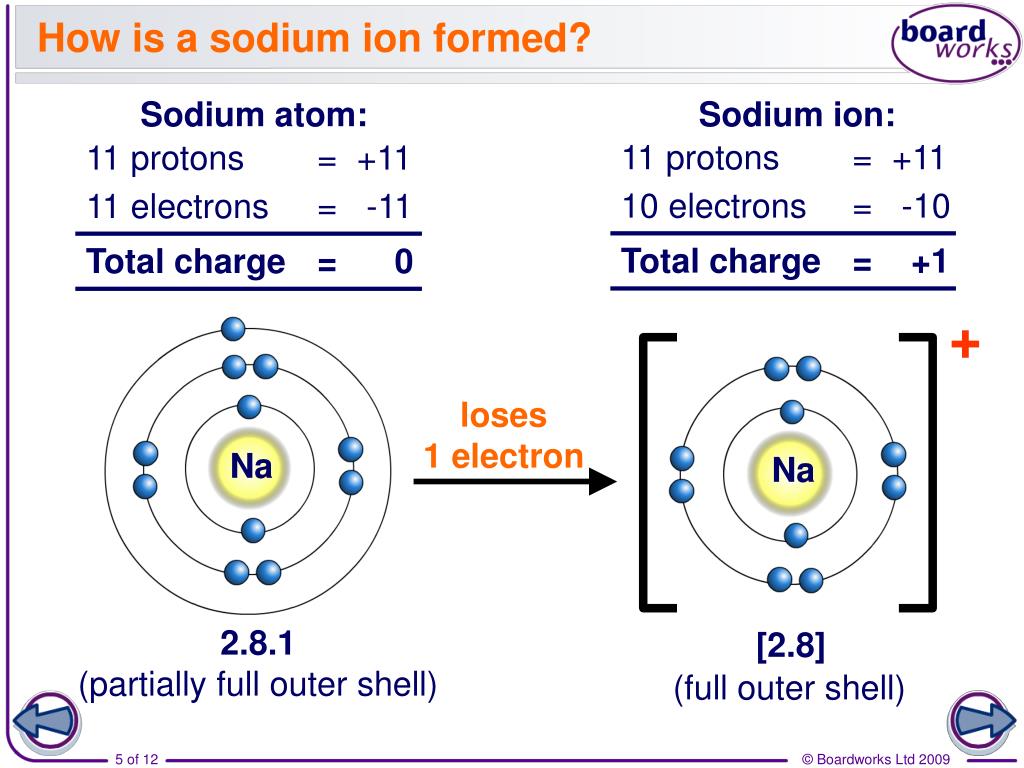
Ions Are Formed When Atoms - Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations;. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic. A positive ion is formed when an element, typically a metal loses 1 or more. Ions are formed when an atome either loses or gains an electron. It has a giant lattice.
Ions are formed when an atome either loses or gains an electron. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic. It has a giant lattice. Positively charged ions are called cations;. A positive ion is formed when an element, typically a metal loses 1 or more. Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons.
Positively charged ions are called cations;. It has a giant lattice. Ions are formed when an atome either loses or gains an electron. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons. A positive ion is formed when an element, typically a metal loses 1 or more. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic.
Ions and Ionic Compounds ppt download
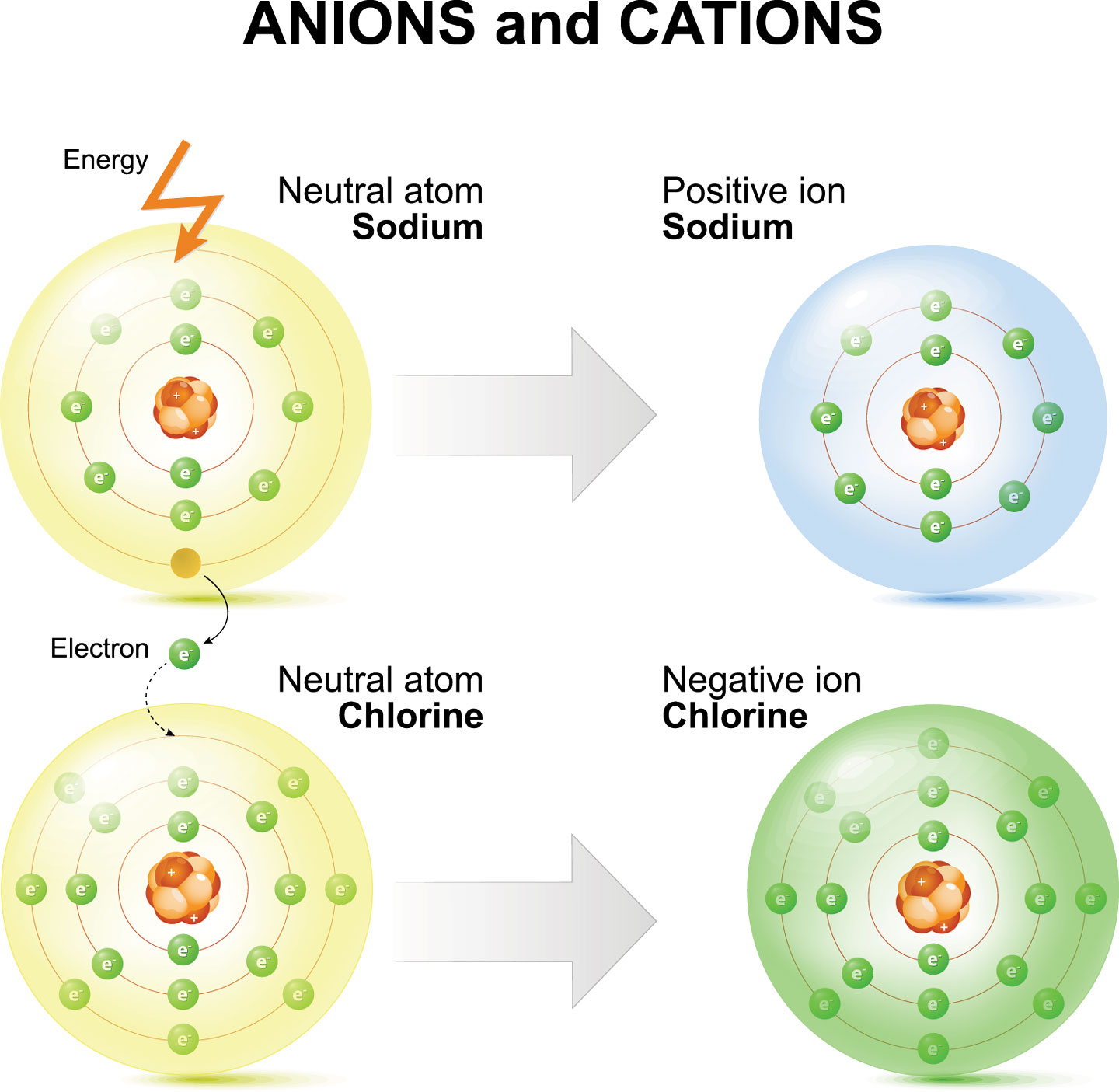
A positive ion is formed when an element, typically a metal loses 1 or more. It has a giant lattice. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic. Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and.
C3 Atoms,elements and compounds ppt video online download
It has a giant lattice. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. A positive ion is formed when an element, typically a metal loses 1 or more. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic. Ions.
Explainer Ions and radicals in our world
Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons. A positive ion is formed when an element, typically a metal loses 1 or more. Ions are formed when an atome either loses or gains an electron. It has a giant lattice. Opposite electric charges are pulled towards one another by.
Ionic Compounds and Metals Bonding and Properties Chapter
Positively charged ions are called cations;. A positive ion is formed when an element, typically a metal loses 1 or more. It has a giant lattice. Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions.
Unit 4 Ions Two ions are talking to each other in solution. ppt download
Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons. Ions are formed when an atome either loses or gains an electron. Opposite electric charges are pulled towards one another by electrostatic force, so.
Ionic & Metallic Bonding ppt download
A positive ion is formed when an element, typically a metal loses 1 or more. Ions are formed when an atome either loses or gains an electron. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic. Positively charged ions are called cations;. Ions are atoms that have.
PPT How do atoms form ions? PowerPoint Presentation, free download
Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic. Ions are atoms that have a positive or negative charge because they have unequal numbers of protons and electrons. A positive ion is formed when an element, typically a metal loses 1 or more. It has a giant.
Atomic Structure and the Periodic Table ppt download
Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. A positive ion is formed when an element, typically a metal loses 1 or more. It has a giant lattice. Ions are formed when an atome either loses or gains an electron. Opposite electric charges are pulled towards one another by electrostatic force,.
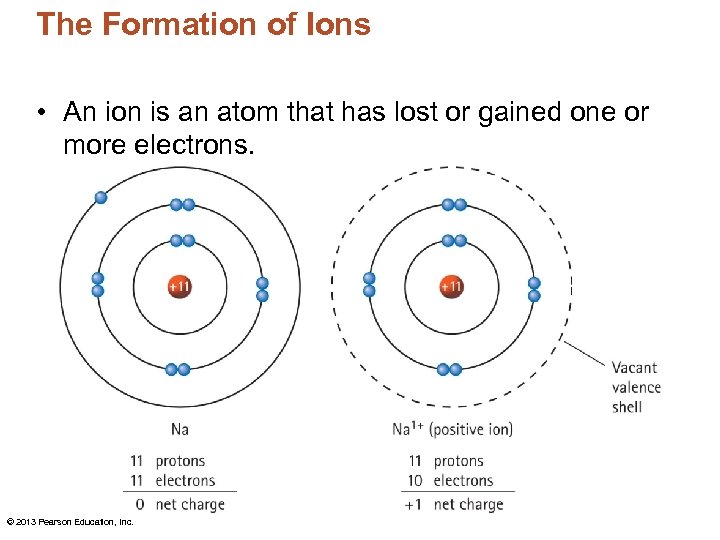
The Formation of Ions An ion is
A positive ion is formed when an element, typically a metal loses 1 or more. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic. It has a giant lattice. Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively.
PPT Ions PowerPoint Presentation, free download ID6738771
Ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations;. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic. A positive ion is formed when an element, typically a metal loses 1 or more..
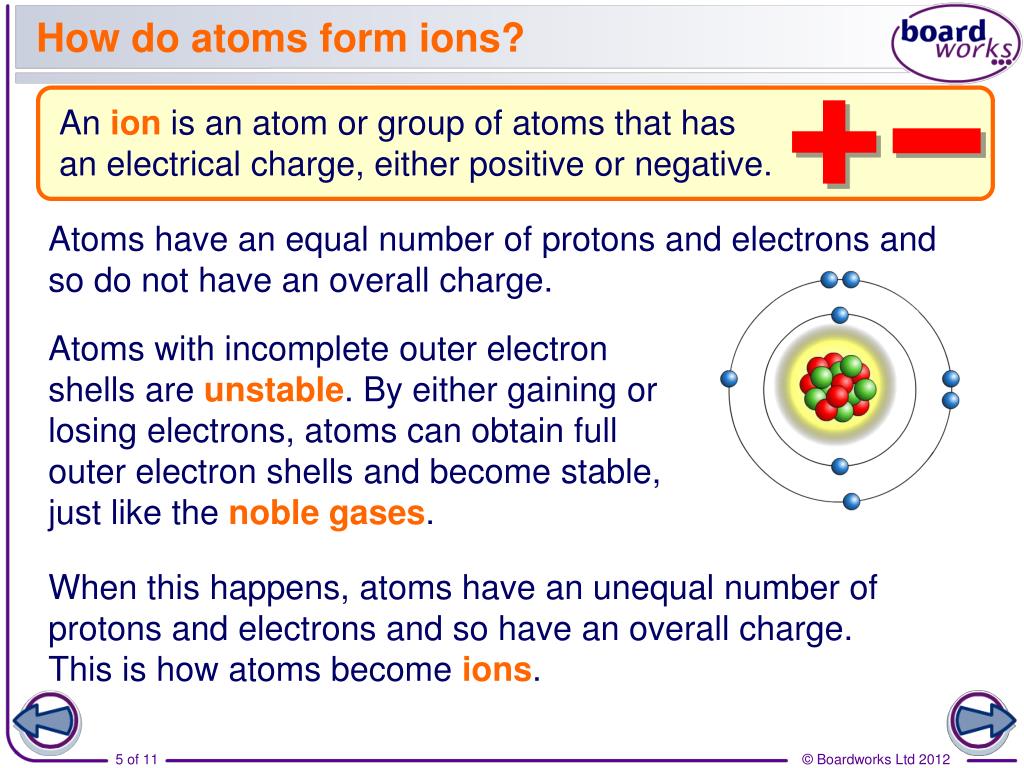
Ions Are Atoms That Have A Positive Or Negative Charge Because They Have Unequal Numbers Of Protons And Electrons.
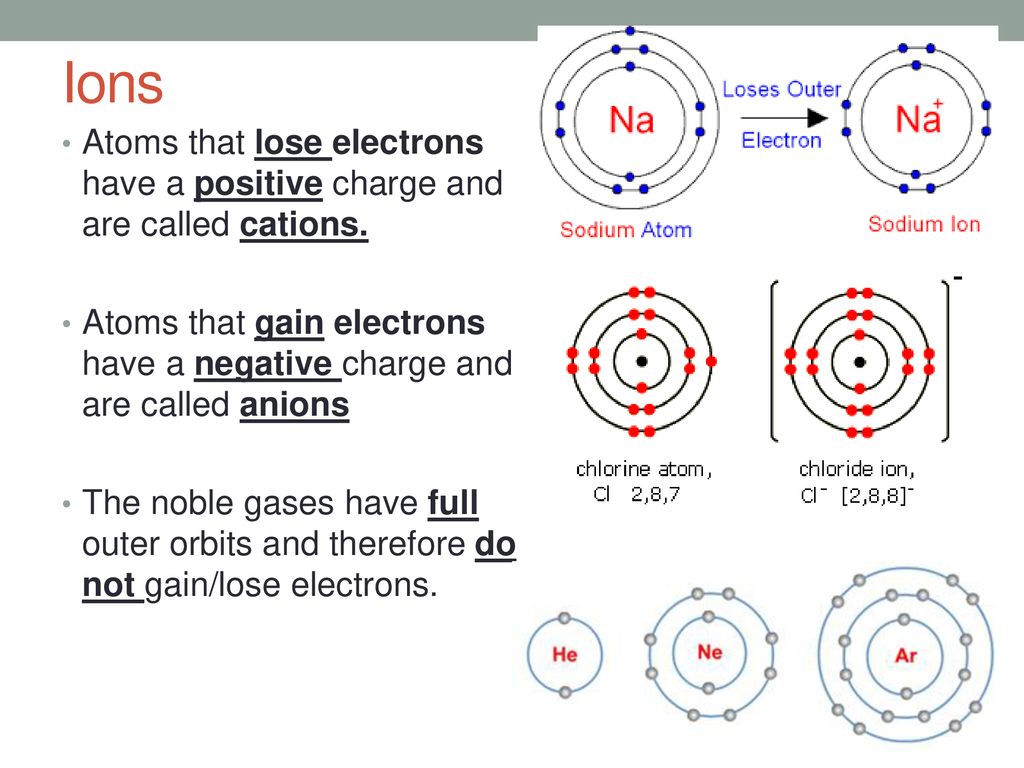
Positively charged ions are called cations;. Ions are formed when an atome either loses or gains an electron. A positive ion is formed when an element, typically a metal loses 1 or more. It has a giant lattice.
Ion, Any Atom Or Group Of Atoms That Bears One Or More Positive Or Negative Electrical Charges.
Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic.

..jpg)

.jpg)