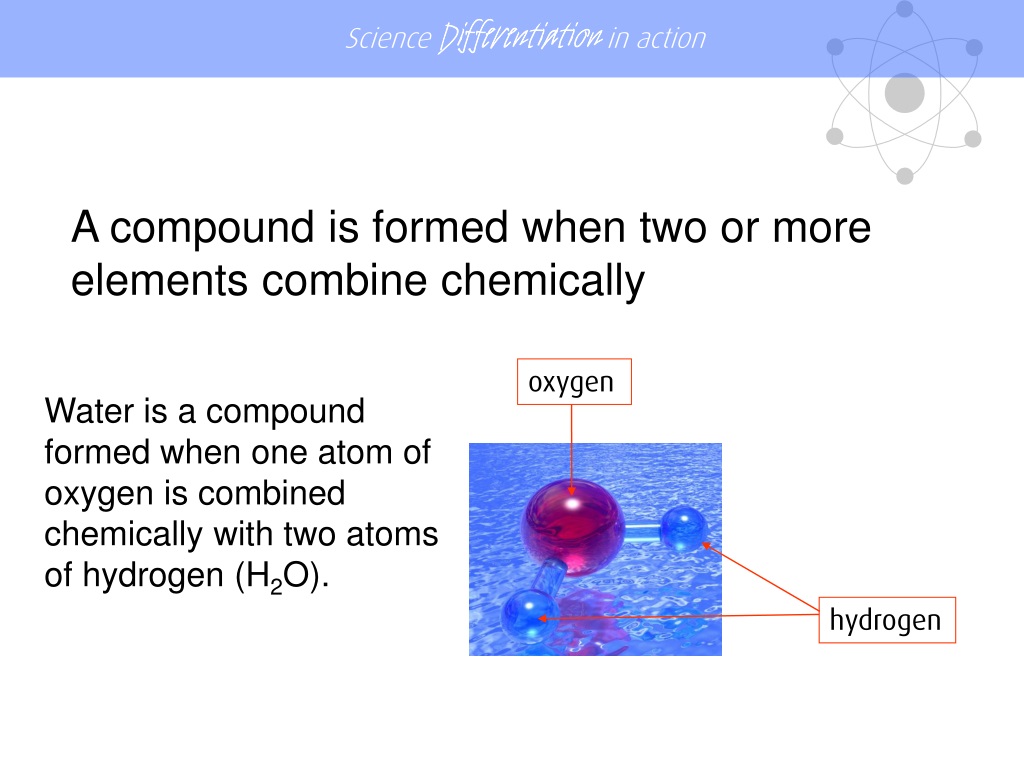
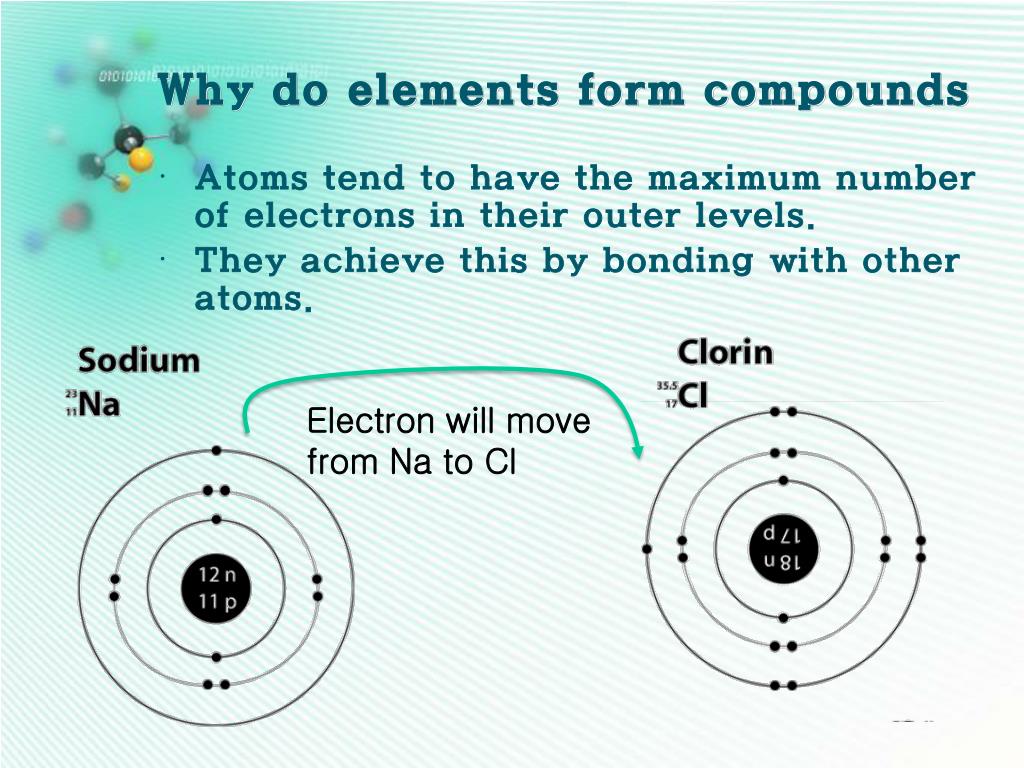
How Is A Compound Formed - The chemical formula for the compound formed between scandium (iii) and the bromate ion is sc (bro3)3. The compound formed is calcium nitrate, ca(no3)2. It is formed when one potassium ion (k+). The ionic compound formed when cs and o react is cesium oxide with the formula cs2o. In this compound, cesium (cs) donates one. The compound formed from the ions of potassium and chlorine is potassium chloride (kcl). Magnesium, with a 2+ charge, forms an ion while. The formula for the ionic compound formed between magnesium and chlorine is mgcl2.
In this compound, cesium (cs) donates one. It is formed when one potassium ion (k+). The compound formed is calcium nitrate, ca(no3)2. The chemical formula for the compound formed between scandium (iii) and the bromate ion is sc (bro3)3. The compound formed from the ions of potassium and chlorine is potassium chloride (kcl). The ionic compound formed when cs and o react is cesium oxide with the formula cs2o. Magnesium, with a 2+ charge, forms an ion while. The formula for the ionic compound formed between magnesium and chlorine is mgcl2.
It is formed when one potassium ion (k+). In this compound, cesium (cs) donates one. Magnesium, with a 2+ charge, forms an ion while. The formula for the ionic compound formed between magnesium and chlorine is mgcl2. The compound formed from the ions of potassium and chlorine is potassium chloride (kcl). The chemical formula for the compound formed between scandium (iii) and the bromate ion is sc (bro3)3. The compound formed is calcium nitrate, ca(no3)2. The ionic compound formed when cs and o react is cesium oxide with the formula cs2o.
Chemistry The Study of Matter ppt video online download
It is formed when one potassium ion (k+). The chemical formula for the compound formed between scandium (iii) and the bromate ion is sc (bro3)3. In this compound, cesium (cs) donates one. The formula for the ionic compound formed between magnesium and chlorine is mgcl2. The ionic compound formed when cs and o react is cesium oxide with the formula.
Compound Turbo 12 Valve
The ionic compound formed when cs and o react is cesium oxide with the formula cs2o. The chemical formula for the compound formed between scandium (iii) and the bromate ion is sc (bro3)3. The compound formed is calcium nitrate, ca(no3)2. The formula for the ionic compound formed between magnesium and chlorine is mgcl2. In this compound, cesium (cs) donates one.
PPT Unit 7 Section 1 Notes PowerPoint Presentation, free download
It is formed when one potassium ion (k+). Magnesium, with a 2+ charge, forms an ion while. The compound formed is calcium nitrate, ca(no3)2. The chemical formula for the compound formed between scandium (iii) and the bromate ion is sc (bro3)3. The formula for the ionic compound formed between magnesium and chlorine is mgcl2.
Polyatomic Compounds. ppt download
Magnesium, with a 2+ charge, forms an ion while. The ionic compound formed when cs and o react is cesium oxide with the formula cs2o. It is formed when one potassium ion (k+). The compound formed from the ions of potassium and chlorine is potassium chloride (kcl). The compound formed is calcium nitrate, ca(no3)2.
What are Ionic Compounds and how they are formed?
Magnesium, with a 2+ charge, forms an ion while. The compound formed is calcium nitrate, ca(no3)2. It is formed when one potassium ion (k+). The formula for the ionic compound formed between magnesium and chlorine is mgcl2. In this compound, cesium (cs) donates one.
What is a compound? a pure substance made up of two or more types of
The formula for the ionic compound formed between magnesium and chlorine is mgcl2. In this compound, cesium (cs) donates one. The ionic compound formed when cs and o react is cesium oxide with the formula cs2o. The compound formed from the ions of potassium and chlorine is potassium chloride (kcl). The chemical formula for the compound formed between scandium (iii).
PPT Lesson Mixtures & Compounds PowerPoint Presentation, free
In this compound, cesium (cs) donates one. It is formed when one potassium ion (k+). The ionic compound formed when cs and o react is cesium oxide with the formula cs2o. The compound formed from the ions of potassium and chlorine is potassium chloride (kcl). The chemical formula for the compound formed between scandium (iii) and the bromate ion is.
Chemical Bonds and forming Compounds. How is a Compound formed? A
The chemical formula for the compound formed between scandium (iii) and the bromate ion is sc (bro3)3. The compound formed is calcium nitrate, ca(no3)2. Magnesium, with a 2+ charge, forms an ion while. The formula for the ionic compound formed between magnesium and chlorine is mgcl2. The ionic compound formed when cs and o react is cesium oxide with the.
Properties of Ionic and molecular compounds ppt download
It is formed when one potassium ion (k+). The formula for the ionic compound formed between magnesium and chlorine is mgcl2. The compound formed is calcium nitrate, ca(no3)2. In this compound, cesium (cs) donates one. The compound formed from the ions of potassium and chlorine is potassium chloride (kcl).
PPT Elements and compounds PowerPoint Presentation, free download
The compound formed is calcium nitrate, ca(no3)2. The chemical formula for the compound formed between scandium (iii) and the bromate ion is sc (bro3)3. In this compound, cesium (cs) donates one. It is formed when one potassium ion (k+). The ionic compound formed when cs and o react is cesium oxide with the formula cs2o.
In This Compound, Cesium (Cs) Donates One.
The formula for the ionic compound formed between magnesium and chlorine is mgcl2. It is formed when one potassium ion (k+). The compound formed is calcium nitrate, ca(no3)2. The compound formed from the ions of potassium and chlorine is potassium chloride (kcl).
Magnesium, With A 2+ Charge, Forms An Ion While.
The chemical formula for the compound formed between scandium (iii) and the bromate ion is sc (bro3)3. The ionic compound formed when cs and o react is cesium oxide with the formula cs2o.