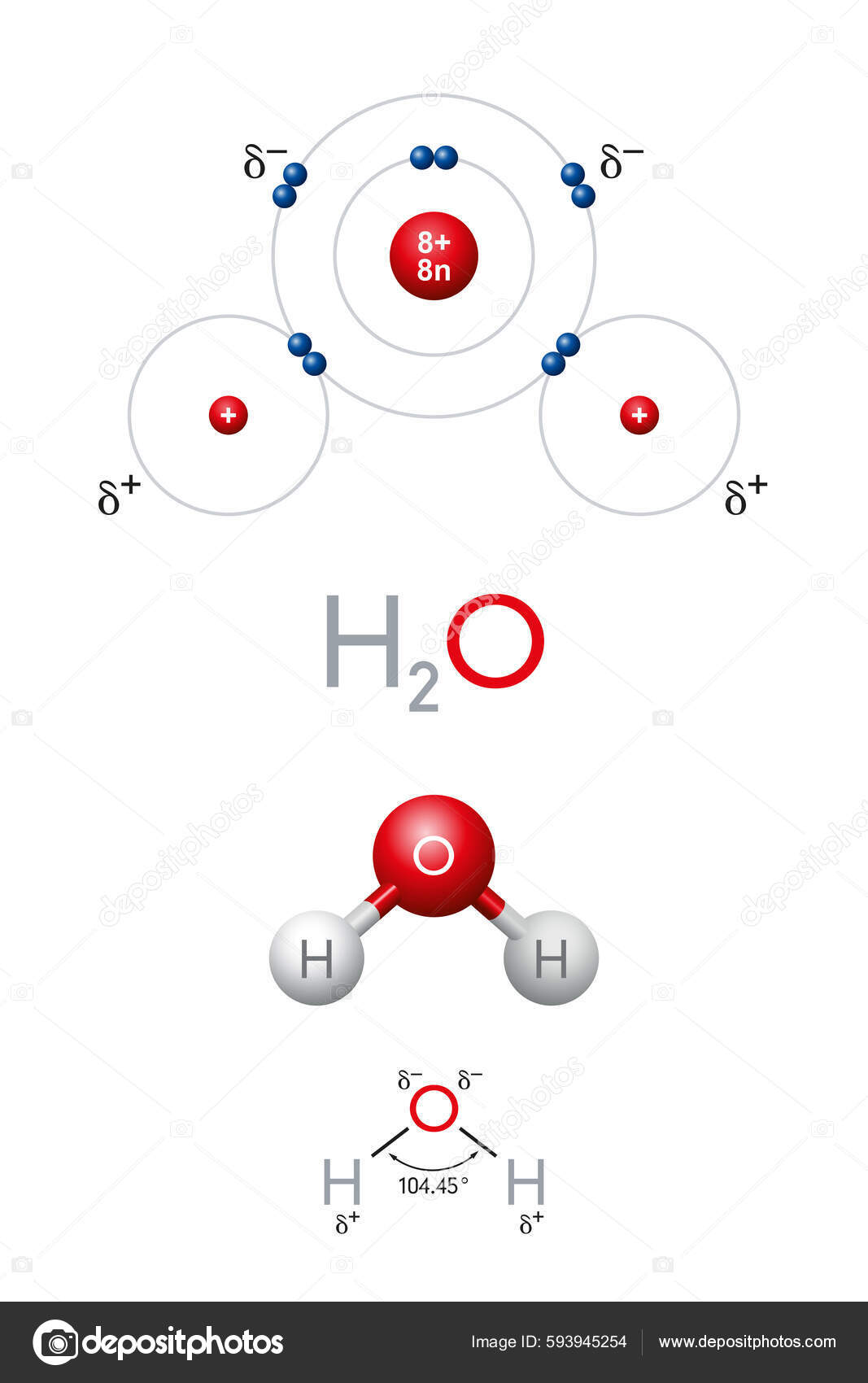
H2O Safety Data Sheet - The clue lies in the name itself. What is this gas if at stp 1l of this gas weighs 1.34g?. An organic compound was taken for combustion.14.77 g was burned in oxygen.the result of combustion gave 43.26g of co2 &12.40g of. Why isn't a mixture of a strong acid and its. Double displacement reactions occur when two ionic compounds react, or when an ionic compound. Could a buffered solution be made by mixing aqueous solutions of hcl and naoh? If the gases are ideal what is the pressure in the cylinder at ambient temperature?. A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. After burning 1.5g of some gas, 4.4g of co2 and 2.7g of h2o were produced.
An organic compound was taken for combustion.14.77 g was burned in oxygen.the result of combustion gave 43.26g of co2 &12.40g of. The clue lies in the name itself. A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. Double displacement reactions occur when two ionic compounds react, or when an ionic compound. What is this gas if at stp 1l of this gas weighs 1.34g?. Could a buffered solution be made by mixing aqueous solutions of hcl and naoh? After burning 1.5g of some gas, 4.4g of co2 and 2.7g of h2o were produced. Why isn't a mixture of a strong acid and its. If the gases are ideal what is the pressure in the cylinder at ambient temperature?.
An organic compound was taken for combustion.14.77 g was burned in oxygen.the result of combustion gave 43.26g of co2 &12.40g of. What is this gas if at stp 1l of this gas weighs 1.34g?. Could a buffered solution be made by mixing aqueous solutions of hcl and naoh? A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. After burning 1.5g of some gas, 4.4g of co2 and 2.7g of h2o were produced. Double displacement reactions occur when two ionic compounds react, or when an ionic compound. If the gases are ideal what is the pressure in the cylinder at ambient temperature?. Why isn't a mixture of a strong acid and its. The clue lies in the name itself.
Chemical formula of water H2O Stock Photo Alamy
A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. What is this gas if at stp 1l of this gas weighs 1.34g?. An organic compound was taken for combustion.14.77 g was burned in oxygen.the result of combustion gave 43.26g of co2 &12.40g of. If the gases are ideal what is the pressure in the.
H2o Water Molecule Model Chemical Formula Vector Image, 51 OFF
An organic compound was taken for combustion.14.77 g was burned in oxygen.the result of combustion gave 43.26g of co2 &12.40g of. What is this gas if at stp 1l of this gas weighs 1.34g?. After burning 1.5g of some gas, 4.4g of co2 and 2.7g of h2o were produced. The clue lies in the name itself. Why isn't a mixture.
H2o
A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. After burning 1.5g of some gas, 4.4g of co2 and 2.7g of h2o were produced. The clue lies in the name itself. Could a buffered solution be made by mixing aqueous solutions of hcl and naoh? Double displacement reactions occur when two ionic compounds react,.
SimplyScience Wunderstoff H2O
Double displacement reactions occur when two ionic compounds react, or when an ionic compound. What is this gas if at stp 1l of this gas weighs 1.34g?. An organic compound was taken for combustion.14.77 g was burned in oxygen.the result of combustion gave 43.26g of co2 &12.40g of. The clue lies in the name itself. After burning 1.5g of some.
Chemical Formula For Water H2o
Why isn't a mixture of a strong acid and its. A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. Could a buffered solution be made by mixing aqueous solutions of hcl and naoh? An organic compound was taken for combustion.14.77 g was burned in oxygen.the result of combustion gave 43.26g of co2 &12.40g of..
H2o Water Structure
After burning 1.5g of some gas, 4.4g of co2 and 2.7g of h2o were produced. What is this gas if at stp 1l of this gas weighs 1.34g?. Double displacement reactions occur when two ionic compounds react, or when an ionic compound. A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. The clue lies.
H2O, Ejemplo De La Molécula De Agua Ilustración del Vector
Double displacement reactions occur when two ionic compounds react, or when an ionic compound. Could a buffered solution be made by mixing aqueous solutions of hcl and naoh? The clue lies in the name itself. What is this gas if at stp 1l of this gas weighs 1.34g?. If the gases are ideal what is the pressure in the cylinder.
H2o water chemical formula illustration Cut Out Stock Images & Pictures
What is this gas if at stp 1l of this gas weighs 1.34g?. After burning 1.5g of some gas, 4.4g of co2 and 2.7g of h2o were produced. Why isn't a mixture of a strong acid and its. If the gases are ideal what is the pressure in the cylinder at ambient temperature?. Double displacement reactions occur when two ionic.
Chemical formula of water H2O Stock Photo Adobe Stock
A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. What is this gas if at stp 1l of this gas weighs 1.34g?. If the gases are ideal what is the pressure in the cylinder at ambient temperature?. An organic compound was taken for combustion.14.77 g was burned in oxygen.the result of combustion gave 43.26g.
modelo de química de la molécula de agua h2o elementos científicos
If the gases are ideal what is the pressure in the cylinder at ambient temperature?. The clue lies in the name itself. After burning 1.5g of some gas, 4.4g of co2 and 2.7g of h2o were produced. What is this gas if at stp 1l of this gas weighs 1.34g?. Could a buffered solution be made by mixing aqueous solutions.
What Is This Gas If At Stp 1L Of This Gas Weighs 1.34G?.
The clue lies in the name itself. Why isn't a mixture of a strong acid and its. An organic compound was taken for combustion.14.77 g was burned in oxygen.the result of combustion gave 43.26g of co2 &12.40g of. Double displacement reactions occur when two ionic compounds react, or when an ionic compound.
Could A Buffered Solution Be Made By Mixing Aqueous Solutions Of Hcl And Naoh?
After burning 1.5g of some gas, 4.4g of co2 and 2.7g of h2o were produced. A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. If the gases are ideal what is the pressure in the cylinder at ambient temperature?.