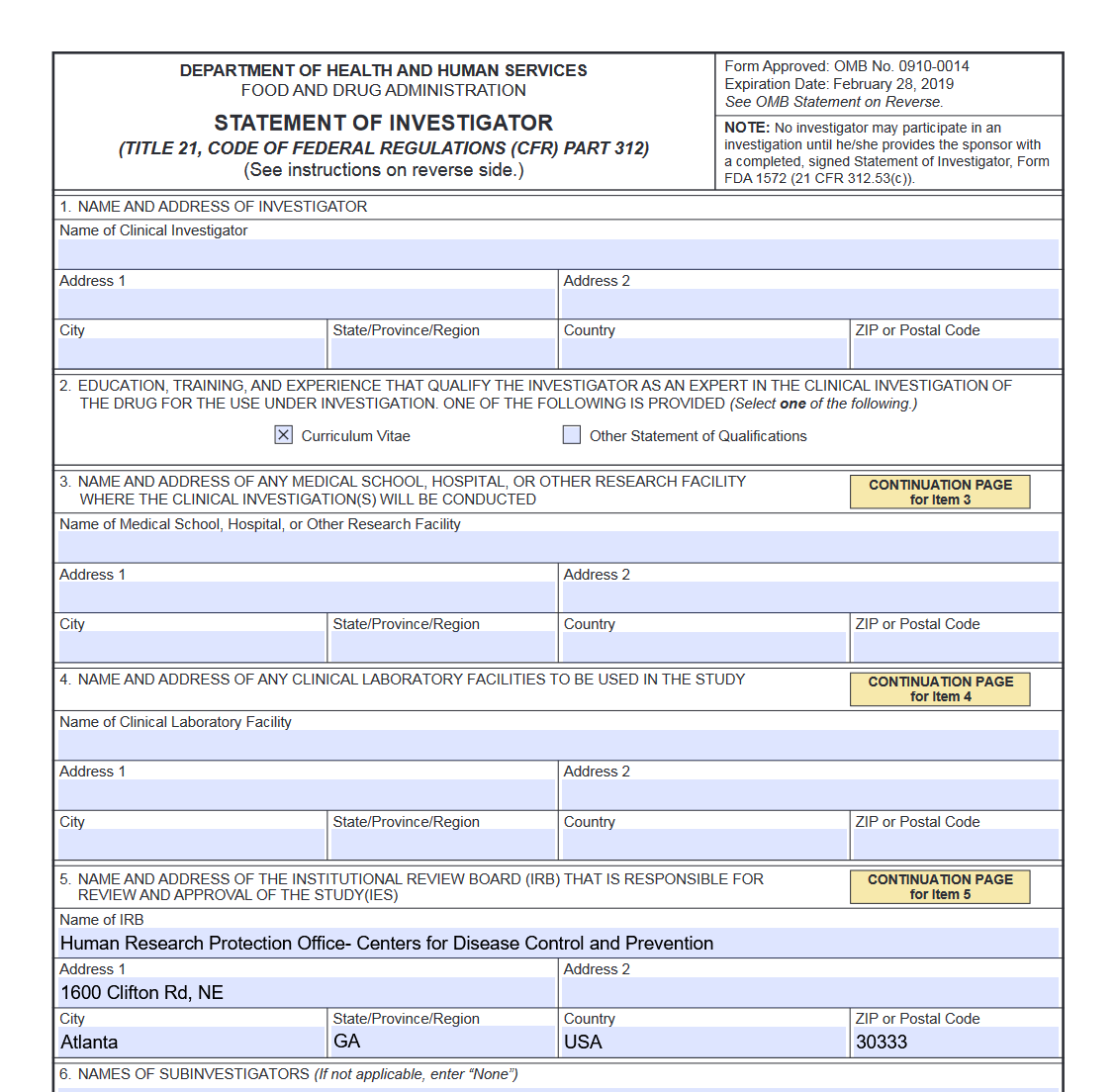
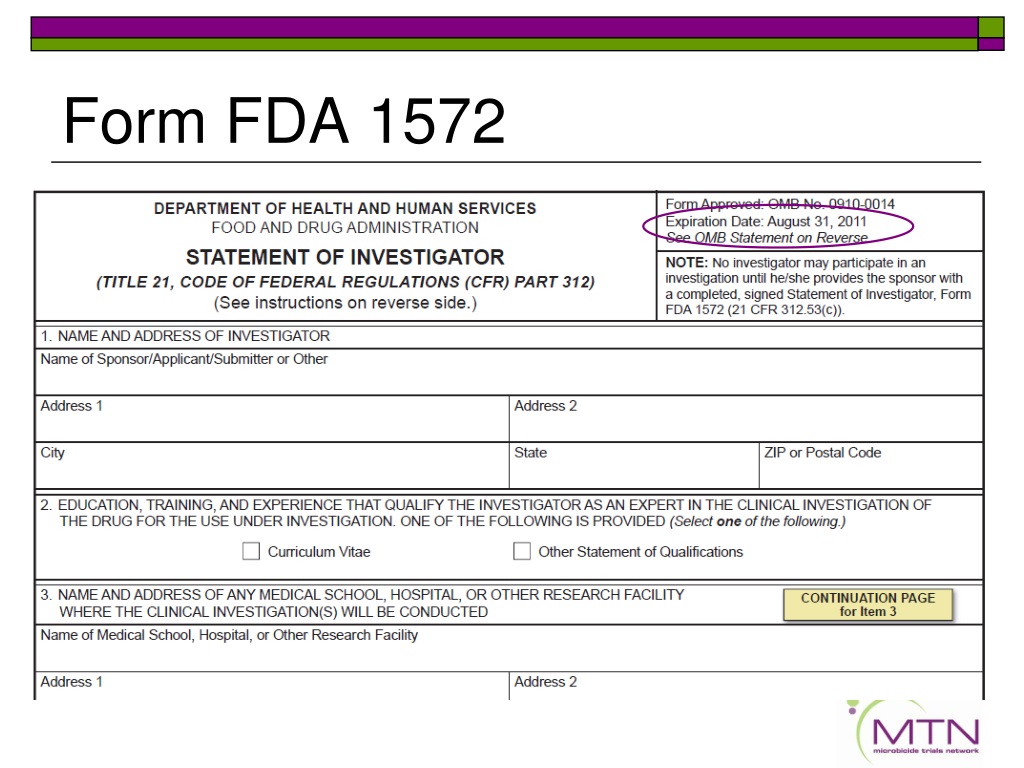
Form 1572 - For other fda forms, visit the fda forms page. This page provides links to commonly used clinical trial forms relevant to clinical trials. The purpose of this guidance is to inform division of microbiology and infectious diseases (dmid) staff, extramural investigators, site staff, and. What is the fda form 1572? The sponsor must obtain a completed and signed 1572 before permitting an. When must the form be completed and signed by the investigator? Form required for clinical trials involving investigational drugs and biologics. The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and.
The sponsor must obtain a completed and signed 1572 before permitting an. Form required for clinical trials involving investigational drugs and biologics. When must the form be completed and signed by the investigator? The purpose of this guidance is to inform division of microbiology and infectious diseases (dmid) staff, extramural investigators, site staff, and. This page provides links to commonly used clinical trial forms relevant to clinical trials. The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. What is the fda form 1572? For other fda forms, visit the fda forms page.
This page provides links to commonly used clinical trial forms relevant to clinical trials. What is the fda form 1572? The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. Form required for clinical trials involving investigational drugs and biologics. The purpose of this guidance is to inform division of microbiology and infectious diseases (dmid) staff, extramural investigators, site staff, and. For other fda forms, visit the fda forms page. When must the form be completed and signed by the investigator? The sponsor must obtain a completed and signed 1572 before permitting an.
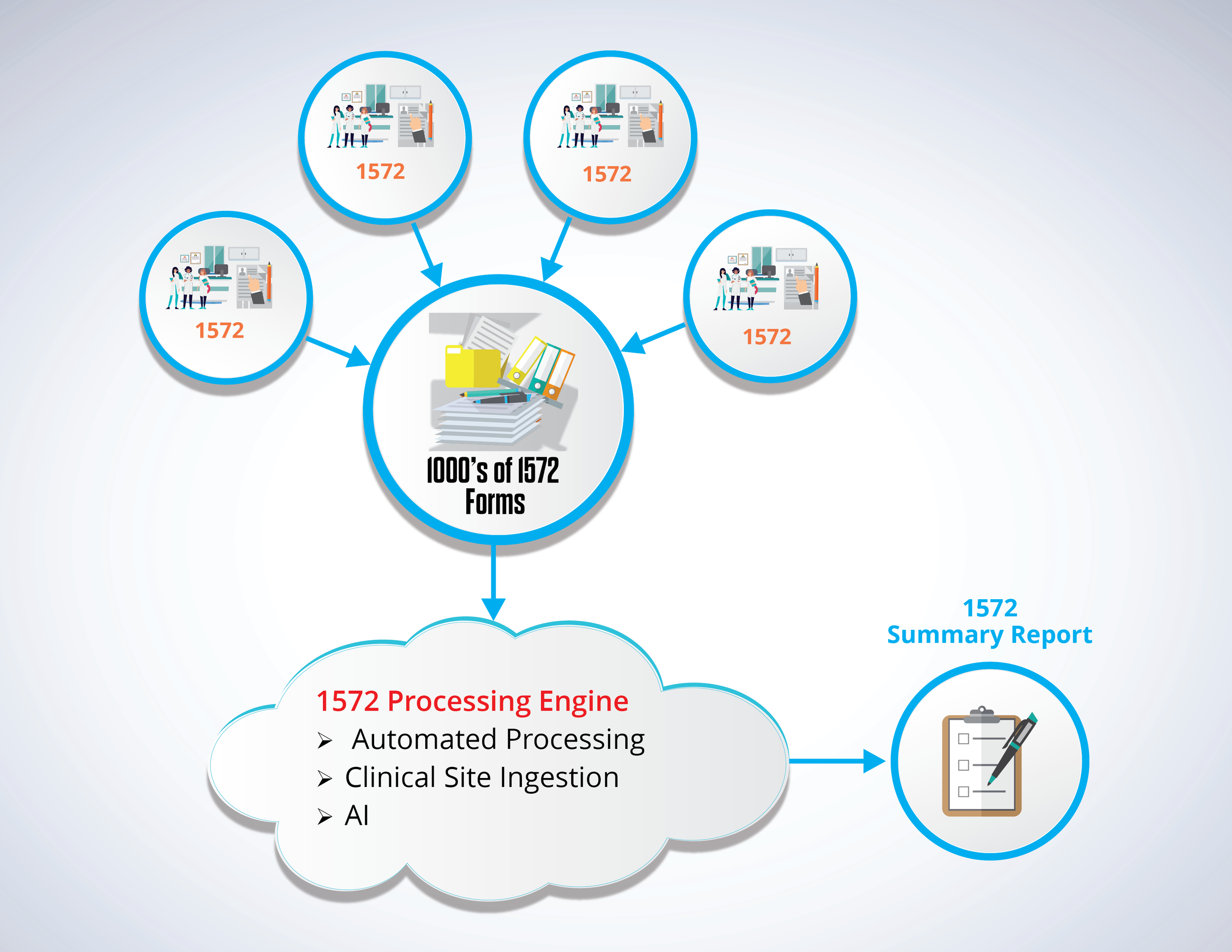
Streamlined Solution for FDA Form 1572 Summary Report Processing
Form required for clinical trials involving investigational drugs and biologics. The sponsor must obtain a completed and signed 1572 before permitting an. When must the form be completed and signed by the investigator? The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. The purpose of.
Top Fda Form 1572 Templates free to download in PDF format
The sponsor must obtain a completed and signed 1572 before permitting an. For other fda forms, visit the fda forms page. What is the fda form 1572? The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. This page provides links to commonly used clinical trial.
Fillable Fda 1572 Form Special Condition Consideration Form printable
This page provides links to commonly used clinical trial forms relevant to clinical trials. When must the form be completed and signed by the investigator? The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. For other fda forms, visit the fda forms page. The sponsor.
PPT IND Basics in OCTGT PowerPoint Presentation, free download ID
The purpose of this guidance is to inform division of microbiology and infectious diseases (dmid) staff, extramural investigators, site staff, and. When must the form be completed and signed by the investigator? This page provides links to commonly used clinical trial forms relevant to clinical trials. For other fda forms, visit the fda forms page. What is the fda form.
The Form FDA 1572 A Reference Guide for Clinical Researchers, Sponsors
This page provides links to commonly used clinical trial forms relevant to clinical trials. When must the form be completed and signed by the investigator? Form required for clinical trials involving investigational drugs and biologics. What is the fda form 1572? The sponsor must obtain a completed and signed 1572 before permitting an.
PPT Clinical Investigator Responsibilities Regulations and
For other fda forms, visit the fda forms page. Form required for clinical trials involving investigational drugs and biologics. When must the form be completed and signed by the investigator? What is the fda form 1572? The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and.
FDA Form 1572 Statement of Investigator Forms Docs 2023
The sponsor must obtain a completed and signed 1572 before permitting an. When must the form be completed and signed by the investigator? The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. For other fda forms, visit the fda forms page. The purpose of this.
Information Sheet, Guidance for Sponsors, Clinical Investigators, and
When must the form be completed and signed by the investigator? The sponsor must obtain a completed and signed 1572 before permitting an. The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and. The purpose of this guidance is to inform division of microbiology and infectious.
Fda 1572 Template
This page provides links to commonly used clinical trial forms relevant to clinical trials. The sponsor must obtain a completed and signed 1572 before permitting an. What is the fda form 1572? When must the form be completed and signed by the investigator? The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to.
Instructions For Filling Out Form Fda 1572 Statement Of Investigator
Form required for clinical trials involving investigational drugs and biologics. What is the fda form 1572? The purpose of this guidance is to inform division of microbiology and infectious diseases (dmid) staff, extramural investigators, site staff, and. For other fda forms, visit the fda forms page. The sponsor must obtain a completed and signed 1572 before permitting an.
For Other Fda Forms, Visit The Fda Forms Page.
Form required for clinical trials involving investigational drugs and biologics. When must the form be completed and signed by the investigator? The sponsor must obtain a completed and signed 1572 before permitting an. The statement of investigator, form fda 1572, is an agreement signed by the principal investigator (pi) to provide certain information to the sponsor and.
This Page Provides Links To Commonly Used Clinical Trial Forms Relevant To Clinical Trials.
The purpose of this guidance is to inform division of microbiology and infectious diseases (dmid) staff, extramural investigators, site staff, and. What is the fda form 1572?