When K+ And Cl- Forms An Ionic Bond - Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? When salts dissolve in water, the anions, cations, and strongly polar water molecules undergo hydration reactions, resulting in the. As the electrons in the 3rd shell of k are not involved in bonding, it. I came across this question: The definition of a valence electron is an electron involved in bonding. 2) what happens to the orbitals, and thus to atoms or ions. An example question would be: 1) count the electrons (all of them) in each atom or ion. Think of it this way: Which of the following is bigger?
2) what happens to the orbitals, and thus to atoms or ions. I came across this question: As the electrons in the 3rd shell of k are not involved in bonding, it. An example question would be: 1) count the electrons (all of them) in each atom or ion. When salts dissolve in water, the anions, cations, and strongly polar water molecules undergo hydration reactions, resulting in the. Think of it this way: Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? Which of the following is bigger? The definition of a valence electron is an electron involved in bonding.
Think of it this way: I came across this question: Which of the following is bigger? 1) count the electrons (all of them) in each atom or ion. As the electrons in the 3rd shell of k are not involved in bonding, it. An example question would be: When salts dissolve in water, the anions, cations, and strongly polar water molecules undergo hydration reactions, resulting in the. The definition of a valence electron is an electron involved in bonding. Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? 2) what happens to the orbitals, and thus to atoms or ions.
Chapter 3 Lecture Outline ppt download
Which of the following is bigger? An example question would be: Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? I came across this question: 2) what happens to the orbitals, and thus to atoms or ions.
Chemical Bonding. ppt download
1) count the electrons (all of them) in each atom or ion. As the electrons in the 3rd shell of k are not involved in bonding, it. Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? Think of it this way: When salts dissolve in water, the anions, cations, and strongly polar.
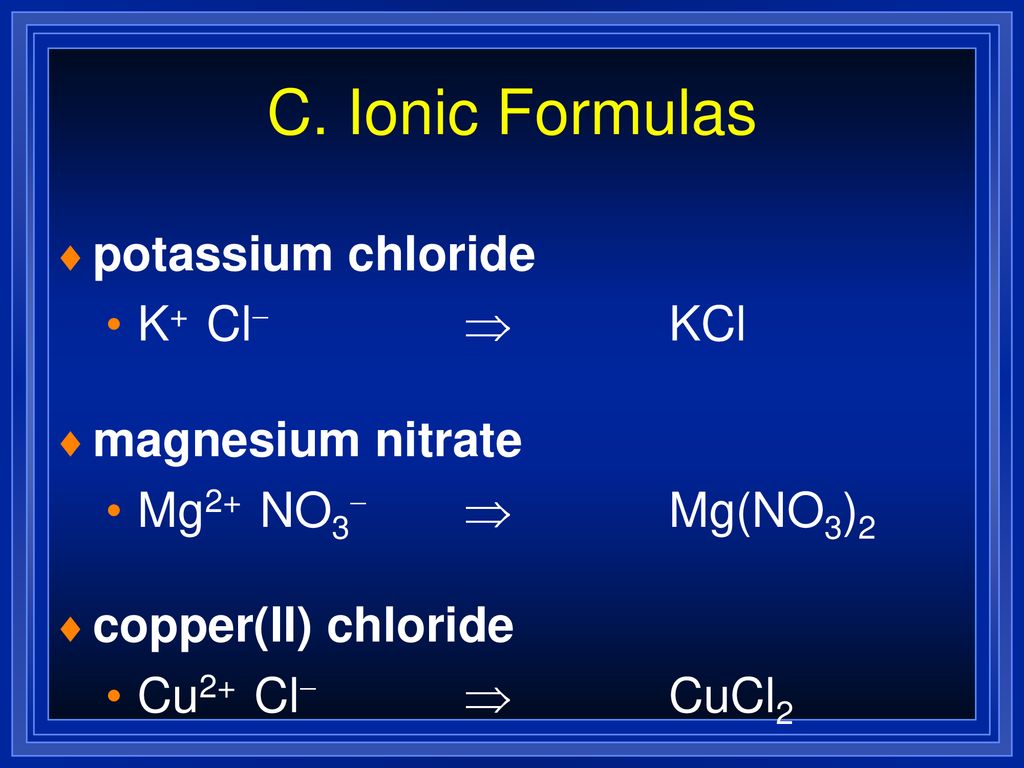
5. Formulas and Names of Ionic Compounds ppt download
The definition of a valence electron is an electron involved in bonding. When salts dissolve in water, the anions, cations, and strongly polar water molecules undergo hydration reactions, resulting in the. 1) count the electrons (all of them) in each atom or ion. An example question would be: Which of the following is bigger?
“Ionic and Metallic Bonding” ppt download
I came across this question: When salts dissolve in water, the anions, cations, and strongly polar water molecules undergo hydration reactions, resulting in the. As the electrons in the 3rd shell of k are not involved in bonding, it. Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? The definition of a.
P461 Molecules1 MOLECULES BONDS Ionic closed shell (+) or open shell
An example question would be: As the electrons in the 3rd shell of k are not involved in bonding, it. 1) count the electrons (all of them) in each atom or ion. Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? I came across this question:
LEWIS STRUCTURES BONDS IONIC BONDING ppt download
An example question would be: 1) count the electrons (all of them) in each atom or ion. Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? As the electrons in the 3rd shell of k are not involved in bonding, it. The definition of a valence electron is an electron involved in.
Ionic Bonding and Ionic Formulas ppt download
An example question would be: As the electrons in the 3rd shell of k are not involved in bonding, it. Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? 1) count the electrons (all of them) in each atom or ion. 2) what happens to the orbitals, and thus to atoms or.
Chapter 6 Language of Chemistry by Christopher G. Hamaker ppt download
1) count the electrons (all of them) in each atom or ion. An example question would be: As the electrons in the 3rd shell of k are not involved in bonding, it. When salts dissolve in water, the anions, cations, and strongly polar water molecules undergo hydration reactions, resulting in the. I came across this question:
Chemical Bonds Review ionic bond ppt download
1) count the electrons (all of them) in each atom or ion. An example question would be: Think of it this way: Which of the following is bigger? Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy?
Ionic Bonding and Ionic Formulas ppt download
I came across this question: As the electrons in the 3rd shell of k are not involved in bonding, it. An example question would be: Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? Think of it this way:
The Definition Of A Valence Electron Is An Electron Involved In Bonding.
Which of the following is bigger? I came across this question: An example question would be: When salts dissolve in water, the anions, cations, and strongly polar water molecules undergo hydration reactions, resulting in the.
1) Count The Electrons (All Of Them) In Each Atom Or Ion.
As the electrons in the 3rd shell of k are not involved in bonding, it. Think of it this way: Between the species $\ce {ne, na+, mg^2+, ar, k+, $\&$~ca^2+}$, which one has the highest ionization energy? 2) what happens to the orbitals, and thus to atoms or ions.

+Non-metals.jpg)